
Research
Developing Technologies to Comprehend Complex Biological Systems
Our research focuses on developing new bioanalytical tools to study complex biological systems and dissect molecular and cellular mechanisms driving their ensemble behaviors.
The ensemble behavior of biological systems, such as tissues and organs or microbial communities, is governed by the molecular profile of individual biological components as well as their interaction with each other and the environment. For instance, brains comprise multiple types of cells, interacting with each other and the environment to drive complex functions and behaviors. Recent advances in sequencing or antibody-based assays have enabled the molecular profiling of individual cells but intercellular interactions and the detailed functional architecture of cells remain elusive. Understanding the functional network of cells is especially important to understand brain functions and dysfunctions as well as cancer development. To tackle these difficult questions, we innovate imaging, sequencing, and sensing technologies that enable high throughput, high resolution profiling of biological systems and to identify molecular and cellular interactions in tissues. We closely work with neurobiologists, cancer biologists, and computational biologists to unveil how healthy and disease tissues respond to external stimuli and identify inter-cellular regulatory networks that are affected during disease.
The evolution of microorganisms is another focus of our research. Due to their high mutation rate, short life cycle, and selection pressures, viruses exist as heterogeneous, dynamic populations. Although a detailed genomic profile of individual viruses greatly informs the infectivity and pathogenesis of the ensemble population, single virus genome sequencing has not been achieved due to the tiny amount of genomic materials in a single virus particle. We are pioneering a single virus genomics platform for high throughput, unbiased sequencing of individual virus genomes. Single virus genomics will open a wide avenue for new investigations in virus infection and evolution. We closely work with field virologists to obtain virus samples from wildlife animals and to design a widely applicable sequencing platform.
We enable new biological studies by inventing bioanalytical tools. Leveraging our extensive expertise in material chemistry, nanotechnology, optics, and microfluidics, we enable in depth analysis of biological substances with high resolution and with minimal disruption of the native biological context. The followings are the drives of technological innovation in our lab:
Multiscale and multimodal imaging platforms: Establishing a comprehensive map of intact tissue at single molecule resolution
The biology of multicellular organisms is structured at multiple levels. Networks of molecular interactions regulate cell function; Interface of cells with nearby cells and environments further shapes their state and functions; Cells form highly interconnected functional networks organ-wide. Imaging is a powerful tool that probes both the molecular profiles and their spatial architecture. The spatial architecture is strongly tied to molecular and cellular interactions as well as higher-level functional networks. We develop imaging platforms that can access different dimensions of biological information at unprecedented scale and resolution. For instance, we develop a high throughput, single molecule-resolution imaging platform to simultaneously image a large number of RNA, DNA, and protein species. Using the new platforms, we aim to extract molecular and cellular interactions that play important role in systems-level functions. We develop both imaging platforms and data processing methodologies.
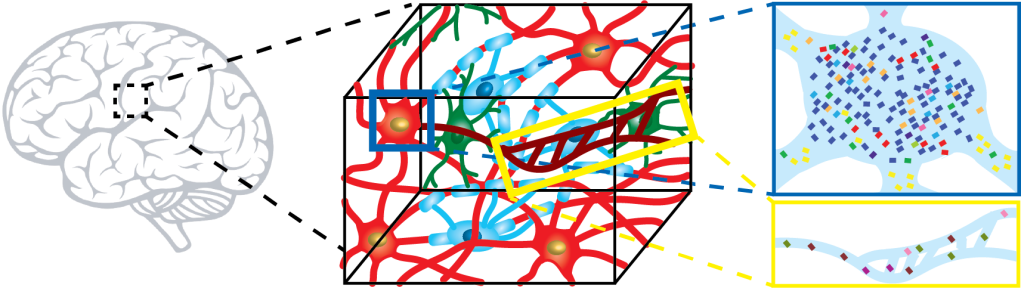
Spatially resolved molecular profiling of intact tissues
Droplet Microfluidics for high throughput, high resolution assays and synthetic biology: Taking both top-down and bottom-up approaches to study complex systems
Droplet microfluidics holds many advantages for the analysis of complex biological systems. First, it compartmentalizes individual cells or molecules into drops, therefore, allows unbiased profiling of each analyte. For instance, in microbial populations, variants with high fitness often dominate the population over time. By compartmentalizing individual cells into well-isolated droplets, the initial population can be preserved during assays. Similarly, droplets provide isolated and controlled environments for cells and molecular reactions. The small volume of drops ensures high reaction efficiencies with minimal input materials, providing single molecule sensitivity. After performing massively parallelized assays, individual drops can be screened one by one, enabling quantitative analysis at single cell/molecule resolution and phenotype-based sorting. We are developing new microfluidic platforms for high throughput, high resolution assays and synthetic cell production. We also seek to understand the fluid dynamics inside microfluidic channels to enable the precise control of drops and cells.
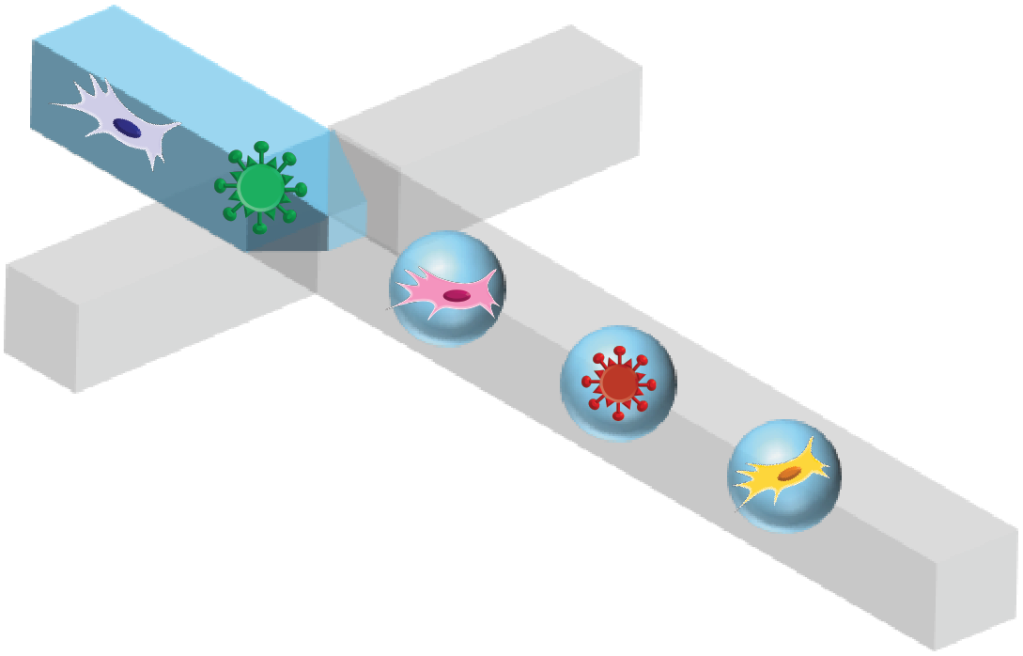

(image credit: DOI:10.1002/adfm.201907182)
Students in our lab will have the opportunity to learn: cellular imaging, droplet microfluidics, materials chemistry, spectroscopic and analytical characterization, single cell/virus genomics, quantum dot synthesis, biostatistics, molecular biology, and many more.
Funding





